Consultation Process and Methods
How is the patient's voice integrated into management's work?
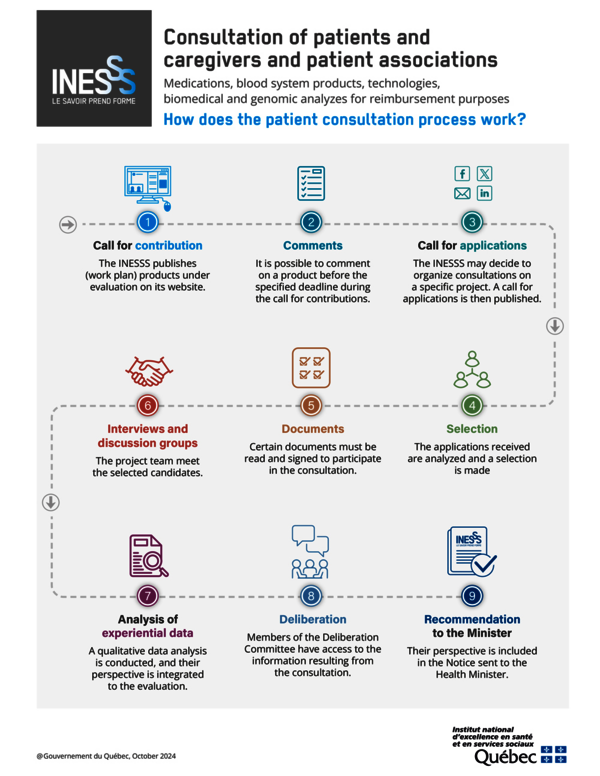
There are two ways to contribute to the work of the Direction de l'évaluation des médicaments et des technologies (DER) for reimbursement purposes: a call for contributions and a call for applications.
Call for contributions: consultation questionnaire
A call for contributions is published indicating the test under evaluation. The consultation period begins as soon as the project is included in the work plan for tests under evaluation. It lasts a total of 30 days. Comments formulated in the questionnaire provided for this purpose must be sent to the INESSS before the end of the period scheduled for each test, so that they can be processed as part of the evaluation process. You can find more information on the Make a Comment on a Test page.
Call for applications: Consultation by Interview or Focus Group
The team responsible for the project may decide to organize consultations (interviews or focus groups) following a call for applications. An invitation to take part in a consultation is posted on the INESSS website, on social networks and through the INESSS Express eNewsletter. Those interested in participating complete the selection questionnaire attached to the invitation. A selection process has been established to ensure diversity of experience and profile.
Only those who respond to the call within the deadline and are selected will be contacted by a member of the project team.
Before the Meeting
An e-mail invitation is sent to those selected, along with the following documents:
- A participant's guide. This guide sets out the themes addressed during the meeting and summarizes the main participation stages.
- A participation agreement document. A participation agreement describing the expectations and commitments between the participants and the INESSS must be signed. It should be noted that the INESSS will do everything in its power to protect the confidentiality of the information provided, in compliance with the applicable laws, including the Act respecting access to documents held by public bodies and the protection of personal information (R.R.Q., c. A-2.1).
- A form for declaring conflicts of interest and roles. The Institute applies its policy on the prevention, identification, evaluation, and management of conflicts of interest and roles for external INESSS collaborators. A form must therefore be completed and signed in application of this policy.
These documents must be read and completed as soon as they are received before taking part in the meeting.
Course of the meeting
Interviews or discussion groups are held virtually on the Microsoft Teams platform. Support is provided for its use. This communication tool is compatible with Chrome, Firefox or Microsoft Edge web browsers. Participants need an Internet connection, and a computer equipped with a microphone (and a camera, optional but preferable).
After the Meeting
A financial compensation form is sent to all participants. The signed form will be processed as soon as possible. Financial compensation will be sent by post.
Mobilizing and integrating the perspectives and knowledge of patients, users, family caregivers and patient associations in evaluation work to the DER
Experiential data from questionnaires, full transcriptions of interviews or focus groups are collected, analyzed and synthesized with a view to methodological rigor and efficiency. These data provide a complementary and necessary perspective for a test, namely the point of view of patients, users, caregivers and their representatives. The findings are used for deliberation purposes and may be incorporated into the INESSS recommendation, forwarded to the Minister of Health and published.
For more details on the processes and methods of DER's participative approach, consult the document provided for this purpose.
If you have any questions about the consultation process for tests evaluated for introductory purposes, please contact our team at the following e-mail address biologie-medicale.genomique@inesss.qc.ca.